For the latest updates, see EPR Release Notices.
For EPR related videos, check out Digital Education's YouTube Channel.
For the latest updates, see EPR Release Notices.
For EPR related videos, check out Digital Education's YouTube Channel.
Paper Order Flag Reminders
Some medications at UHN are considered out-of-scope for EPR and ordered on paper, such as Clinical Trial drugs. The details of these medication orders are written in the paper chart and administration is documented on paper. However, to reduce the patient safety risk of potentially missing these medications, an electronic reminder procedure is ordered in the EPR in addition to writing the order details in the paper chart. This electronic flag serves as a reminder of the medication schedule and also acts as a visual cue for the nurse to check the written order for any changes.
Prior to placing new orders, you must always review the current active orders for the patient to avoid duplication or conflicting orders. These orders can be found by clicking on the Order History tab located within the Order Entry screen.
Note: When ordering medications that utilize the “Paper MAR” reminder, it will not appear in the Order History tab. This will not affect the reminder in the MAR itself. The Paper MAR reminder will continue to appear in the MAR and will be removed if the corresponding medication is discontinued.
Steps for ordering the paper order flag reminder:
1. From within the Order Entry screen, click on the Search tab. Type paper- in the Search Options field and click on the Search button. Select the Clinical Trial medication - see paper procedure from the list. Click on the Add Order button to proceed with the order.
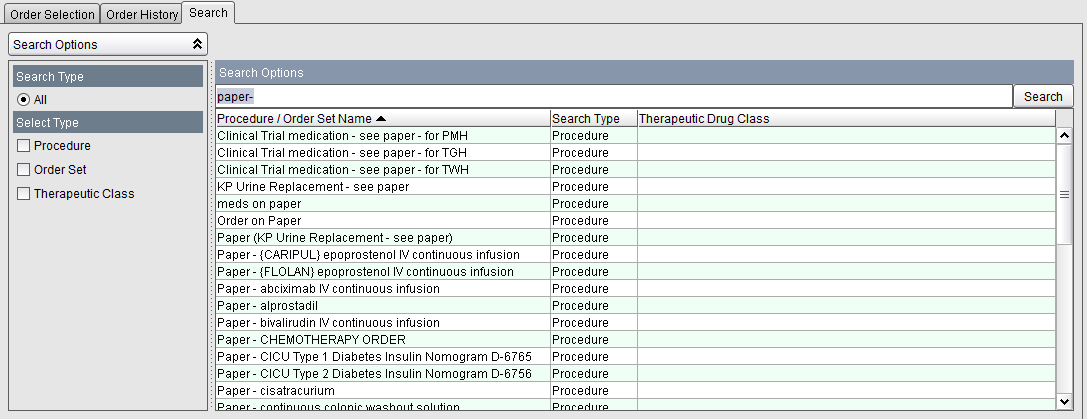
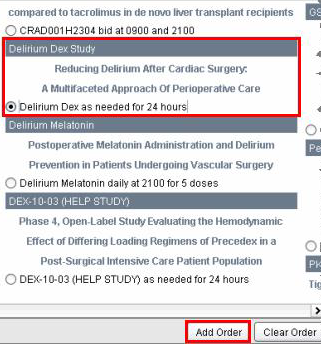
2. Select
the Clinical Trial Medication reminder procedure you wish to order
and then click on the Add Order button.
3. Review the Order Information details and the Start Time of the order. Click on the Accept Order button.
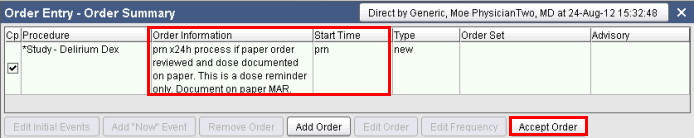
Note: An Add Now event may be added, to have the medication reminder start at the current time, if necessary.
Additional Notes:
The Clinical Trials Medication reminder will be available in the EPR for Clinical Trial medications that have gone through the appropriate approval processes.
The Clinical Trials Medication reminder is ordered by the Principal Investigator or Study Delegate.
Typical order screens have been customized based on the types of studies being conducted at each facility (e.g. TGH and TWH).
Only the When field of the order profile will be modifiable to allow appropriate scheduling of the reminder. The written order will serve as the source documentation for all other order details.
The Clinical Trial Medication reminder should be discontinued when the written order is discontinued in EPR when the written order is discontinued.
The Clinical Trial Medication reminder will be documented in the electronic MAR similar to other medication reminders; however, the paper MAR (or Kardex) must be reviewed to determine actual dose administration information.
Clinical Trial medications will appear at the beginning of the 7 Day Medication History and Medication Transfer Report; however, the paper MAR (or Kardex) must be reviewed to determine actual dose administration information.
Receipt of the Yellow copy of the doctor’s order sheet or notice of EPR flag will begin the study medication procurement and dispensing process.
For further information, please click here.